Home
A comprehensive resource for safe and responsible laser use
US: FDA recommends against using Laserworld and Ray Technologies laser projectors
On November 7 2017, the U.S. Food and Drug Administration issued a Safety Communication “recommending [that] entertainment venues, and other laser light show manufacturers stop using Laserworld and RTI [Ray Technologies International] Class IIIb and Class IV laser projectors because they lack required safety features that protect the user and general public from harmful exposures to high-powered laser radiation. Missing features can include a manual reset mechanism and remote interlock connector. These features prevent unintended laser exposures that can be harmful.”
Laserworld set up a special website, www.cdrh.info, with a statement and information from their viewpoint.
The International Laser Display Association published guidance for ILDA Members and others who are doing shows in the U.S. with Laserworld and RTI projectors.
Laserworld set up a special website, www.cdrh.info, with a statement and information from their viewpoint.
The International Laser Display Association published guidance for ILDA Members and others who are doing shows in the U.S. with Laserworld and RTI projectors.
US: FDA recalls certain X-Laser light show projectors
On November 18 2017, the U.S. Food and Drug Administration announced recall Z-2870-2017 of X-Laser Laser Light Show projector models Aurora, Caliente Aurora, PSX-400, Mobile Beat Max, Mobile Beat Max MKII, X-Beam, and Hawk 500, for failure to comply with performance standard requirements (21 CFR 1040.10(f)) when operated in the user accessible auto and music modes of operation.
FDA listed the following actions:
X-Laser LLC will bring into compliance:
1. All purchasers and associated dealers of affected LLS projector models will be notified by mail and email of their failure to comply with the performance standard. The notification will follow the format and include the information required by 21 CFR 1003.21. Those that do not respond within 14 days will be notified a second time. Those not responding to the second attempt will be notified again every 6 months for the next 2 years. Non-responsive dealers will be ineligible for future orders.
2. All affected LLS projectors will be repaired by removing the auto and music modes from the dipswitch accessible settings, after which, these modes will only be accessible through the DMX connection. These actions, including transportation of the LLS projector, will be made free of charge.
3. All LLS projector models that X-Laser receives, regardless of purpose, will be checked for dipswitch accessible auto or music modes and repaired if needed.
4.Corrective actions will be completed within 120 days of receipt of this letter.
For further questions please call (866) 702-7768.
For additional details from X-Laser, click the “read more” link. Click to read more...
FDA listed the following actions:
X-Laser LLC will bring into compliance:
1. All purchasers and associated dealers of affected LLS projector models will be notified by mail and email of their failure to comply with the performance standard. The notification will follow the format and include the information required by 21 CFR 1003.21. Those that do not respond within 14 days will be notified a second time. Those not responding to the second attempt will be notified again every 6 months for the next 2 years. Non-responsive dealers will be ineligible for future orders.
2. All affected LLS projectors will be repaired by removing the auto and music modes from the dipswitch accessible settings, after which, these modes will only be accessible through the DMX connection. These actions, including transportation of the LLS projector, will be made free of charge.
3. All LLS projector models that X-Laser receives, regardless of purpose, will be checked for dipswitch accessible auto or music modes and repaired if needed.
4.Corrective actions will be completed within 120 days of receipt of this letter.
For further questions please call (866) 702-7768.
For additional details from X-Laser, click the “read more” link. Click to read more...
US: Nationwide recall of Santajoy Christmas laser lights sold at Walmart
The following is from a December 22 2017 Business Wire press release:
On Dec. 22, 2017, Santajoy initiated a nationwide recall of Galaxy Holiday Laser Lights and Northern Lights Holiday Laser Lights. These laser projection products may incorporate a laser having a higher output than intended and fail to comply with FDA performance standard requirements (21 CFR 1040.10 and 1040.11). These higher-power lasers have the potential for eye injury.
Consumers who purchased any of the 5,254 units of Galaxy Holiday Laser Lights and Northern Lights Holiday Laser Lights sold by Walmart between August 1, 2017 and October 25, 2017 should stop using them and return them to any Walmart store for a full refund.
The Galaxy Holiday Laser Lights and Northern Lights Holiday Laser Lights were manufactured from May through September 2017 and distributed from August through October 2017. The affected products sold by Walmart can be identified by the packaging photos and UPC numbers shown below.
Santajoy voluntarily recalled these products after becoming aware that the product presented a potential safety hazard and has notified the FDA of this action. There have been no reports of injury related to the use of these products. Santajoy is notifying the public through this press release, and Walmart is accepting the return of these products for a full refund.
Walmart Stores Inc. distributed these products nationwide. Consumers with questions may contact Walmart via telephone at 1-800-Walmart from 7 a.m. to 9 p.m. CT Monday through Friday or online at www.corporate.walmart.com/recalls for more information.
On Dec. 22, 2017, Santajoy initiated a nationwide recall of Galaxy Holiday Laser Lights and Northern Lights Holiday Laser Lights. These laser projection products may incorporate a laser having a higher output than intended and fail to comply with FDA performance standard requirements (21 CFR 1040.10 and 1040.11). These higher-power lasers have the potential for eye injury.
Consumers who purchased any of the 5,254 units of Galaxy Holiday Laser Lights and Northern Lights Holiday Laser Lights sold by Walmart between August 1, 2017 and October 25, 2017 should stop using them and return them to any Walmart store for a full refund.
The Galaxy Holiday Laser Lights and Northern Lights Holiday Laser Lights were manufactured from May through September 2017 and distributed from August through October 2017. The affected products sold by Walmart can be identified by the packaging photos and UPC numbers shown below.
Santajoy voluntarily recalled these products after becoming aware that the product presented a potential safety hazard and has notified the FDA of this action. There have been no reports of injury related to the use of these products. Santajoy is notifying the public through this press release, and Walmart is accepting the return of these products for a full refund.
Walmart Stores Inc. distributed these products nationwide. Consumers with questions may contact Walmart via telephone at 1-800-Walmart from 7 a.m. to 9 p.m. CT Monday through Friday or online at www.corporate.walmart.com/recalls for more information.


Note: As of January 1 2018, neither laser was listed on the Walmart Product Recalls webpage. The product recall also did not appear to be at FDA’s recalls webpage or enforcement report webpage, as of January 1. The only online source on that date was the December 22 2017 Business Wire press release, or a few publications and news sources such as KCTV that reprinted the Business Wire press release.
US: FDA issues warning about laser toys
25 Nov 2017 -- Categories: Labels and warnings | Media and press | Ways to reduce incidents | SLA news
On November 24 2017, the U.S. Food and Drug Administration issued a “Consumer Update” warning of the dangers of laser toys.
FDA gave these examples of laser toys:
According to the Consumer Update, “Toys with lasers are of particular interest to the FDA because children can be injured by these products. Because they are marketed as toys, parents and kids alike may believe they’re safe to use.”
The FDA had tips for safe use, including:
The FDA’s health warning was referenced in numerous news and publication sources over the 2017 holiday season.
From the FDA Consumer Update, “Laser Toys: How to Keep Kids Safe”. FDA also linked to a 2015 FDA YouTube video on laser pointer safety.
For background, LaserPointerSafety.com has a series of webpages about laser toys which begin with a summary here.
FDA gave these examples of laser toys:
- Lasers mounted on toy guns that can be used for “aiming”;
- Spinning tops that project laser beams while they spin;
- Hand-held lasers used during play as “light sabers”; and
- Lasers intended for entertainment that create optical effects in an open room.
According to the Consumer Update, “Toys with lasers are of particular interest to the FDA because children can be injured by these products. Because they are marketed as toys, parents and kids alike may believe they’re safe to use.”
The FDA had tips for safe use, including:
- Do not aim at persons or animals
- Do not aim at any vehicle, aircraft or shiny surface; or persons playing sports
- Children’s toy lasers should be Class I.
- Children should not be allowed to own or use laser pointers. Pointers are not toys.
- Do not buy or use any laser that emits more than 5 milliwatts.
- See a health care professional in case of a known or suspected laser eye injury.
The FDA’s health warning was referenced in numerous news and publication sources over the 2017 holiday season.
From the FDA Consumer Update, “Laser Toys: How to Keep Kids Safe”. FDA also linked to a 2015 FDA YouTube video on laser pointer safety.
For background, LaserPointerSafety.com has a series of webpages about laser toys which begin with a summary here.
US: Review of 1-watt blue laser with US-required safety features
04 May 2014 -- Categories: Laser pointer review | SLA news
A USD $159 1-watt blue laser, apparently having all required FDA safety features, was reviewed by a gadget blog on May 4 2014.
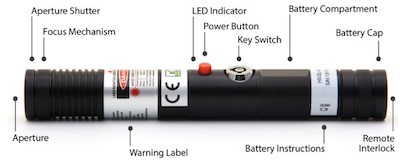
The SKY Technologies Blue Handheld includes a keyswitch, 3-5 second emission delay, remote interlock, and a shutter to cut off the beam, as required by FDA regulations enforced by the agency’s Center for Devices and Radiological Health (CDRH). Under current (May 2014) law, the laser appears to be legal for sale and use in the U.S., assuming the manufacturer also submitted a proper Laser Product Report and has filled all other FDA/CDRH import and paperwork obligations.*
The SKY Technologies Blue Handheld includes a keyswitch, 3-5 second emission delay, remote interlock, and a shutter to cut off the beam, as required by FDA regulations enforced by the agency’s Center for Devices and Radiological Health (CDRH). Under current (May 2014) law, the laser appears to be legal for sale and use in the U.S., assuming the manufacturer also submitted a proper Laser Product Report and has filled all other FDA/CDRH import and paperwork obligations.*
US: FDA proposes defacto ban on selling pointers, handhelds above 5 milliwatts
The U.S. Food and Drug Administration on May 5 2014 will announce its intent to limit laser pointers and handheld lasers to be below 5 milliwatts. If adopted, this action would impose a defacto ban in the U.S. on the sale to consumers of portable, battery powered lasers of 5 mW or more. Currently, such lasers are available for sale in the U.S. at powers of up to 3 watts (3000 milliwatts) which is 600 times the proposed FDA limit.
Although the agency did not give a reason, such bans have been imposed in other countries in response to climbing numbers of laser illuminations of aircraft as well as reports of eye injuries caused by higher-powered consumer lasers.
The proposal would not make it illegal to own or responsibly use portable, battery-powered lasers of 5 mW or more. However, manufacturers could not make or sell these into general commerce in the U.S.
The agency will accept comments for 90 days (until August 2 2014) on the new proposal. FDA will then review the comments. Based on whether it believes any objections or suggestions are valid, the agency could put the guidance into effect (thus imposing their new interpretation), could submit a revised proposal, or could withdraw its proposal.
FDA does not have direct authority over battery powered portable lasers. For example, the words “pointer” and “handheld” laser do not appear in U.S. laser regulations 21 CFR 1040.10 and 1040.11.
Therefore, to regulate these lasers, the May 5 draft proposes an extension of the FDA’s existing authority to regulate surveying, leveling and alignment (“SLA”) lasers. In the May 5 proposal, FDA asserts that the existing definition of SLA lasers also can applied to lasers with the following design characteristics:
According to the FDA, these types of lasers would be affected by the new 5 mW limit:
The FDA's proposed 5 mW limit would NOT apply to lasers with the following design characteristics:
From the FDA’s Surveying, Leveling, or Alignment Laser Products - Draft Guidance for Industry and Food and Drug Administration Staff webpage, published online May 2 2014. This webpage includes the procedure for submitting comments to FDA.The FDA’s PDF version of the draft guidance document is here.
Editorial comment from LaserPointerSafety.com: We have previously published our opinion disagreeing with the FDA’s interpretation of “SLA” lasers. The existing regulations are clear on what constitutes “surveying, leveling or alignment” (SLA) lasers. While we understand the FDA’s intent, in our view, they are going about it the wrong way. They are essentially “making it up” by adding characteristics (size, battery power) which are in no way derived from the clear, existing definition of SLA lasers. As support of this position, we have not found any surveying, leveling or alignment lasers which look the same as the majority of laser pointers and handhelds. This topic is discussed in much greater detail on our page describing FDA authority over laser pointers and handheld lasers.
Although the agency did not give a reason, such bans have been imposed in other countries in response to climbing numbers of laser illuminations of aircraft as well as reports of eye injuries caused by higher-powered consumer lasers.
The proposal would not make it illegal to own or responsibly use portable, battery-powered lasers of 5 mW or more. However, manufacturers could not make or sell these into general commerce in the U.S.
The agency will accept comments for 90 days (until August 2 2014) on the new proposal. FDA will then review the comments. Based on whether it believes any objections or suggestions are valid, the agency could put the guidance into effect (thus imposing their new interpretation), could submit a revised proposal, or could withdraw its proposal.
What lasers are covered by the proposed 5 mW limit?
FDA does not have direct authority over battery powered portable lasers. For example, the words “pointer” and “handheld” laser do not appear in U.S. laser regulations 21 CFR 1040.10 and 1040.11.
Therefore, to regulate these lasers, the May 5 draft proposes an extension of the FDA’s existing authority to regulate surveying, leveling and alignment (“SLA”) lasers. In the May 5 proposal, FDA asserts that the existing definition of SLA lasers also can applied to lasers with the following design characteristics:
- Compact size (i.e. small, lightweight)
- Battery power
- Ergonomic design to permit hand-held use
- An aperture in the laser product's protective housing to transmit laser emission into open space
- Portability to permit use in open spaces or in unrestricted environments
- Features that utilize the laser’s straight line emission for surveying, leveling, or alignment
According to the FDA, these types of lasers would be affected by the new 5 mW limit:
- Laser pointers
- Levels
- Tools incorporating laser guides
- Gun sights
- Target designators
- Night vision illuminators
- Visual disruptors
What lasers are NOT covered by the proposed 5 mW limit?
The FDA's proposed 5 mW limit would NOT apply to lasers with the following design characteristics:
- Predictable, stable power input and output
- High quality power supply and/or power conditioning components
- Adjustability of power and wavelength
- Design that facilitates remote actuation
- Non-portability
- Hard wire connection to power mains
From the FDA’s Surveying, Leveling, or Alignment Laser Products - Draft Guidance for Industry and Food and Drug Administration Staff webpage, published online May 2 2014. This webpage includes the procedure for submitting comments to FDA.The FDA’s PDF version of the draft guidance document is here.
Editorial comment from LaserPointerSafety.com: We have previously published our opinion disagreeing with the FDA’s interpretation of “SLA” lasers. The existing regulations are clear on what constitutes “surveying, leveling or alignment” (SLA) lasers. While we understand the FDA’s intent, in our view, they are going about it the wrong way. They are essentially “making it up” by adding characteristics (size, battery power) which are in no way derived from the clear, existing definition of SLA lasers. As support of this position, we have not found any surveying, leveling or alignment lasers which look the same as the majority of laser pointers and handhelds. This topic is discussed in much greater detail on our page describing FDA authority over laser pointers and handheld lasers.
US: Underwriters Labs offers third-party testing for laser pointer sellers
Underwriters Laboratories announced on October 17 2013 that it will offer third-party testing of laser pointers for manufacturers and retailers. UL said this came in response to increasing laser pointer incidents and mislabeling. In February 2013, the National Institute of Standards and Technology reported that most laser pointers they tested were not in compliance with U.S. laser regulations.
These regulations, 21 CFR 1040.10 and 1040.11, require laser product manufacturers only to self-certify to the Food and Drug Administration that their products meet safety standards. Once the FDA’s Center for Devices and Radiological Health reviews and acknowledges the certification, the laser product can be marketed in the United States.
UL is providing a third-party, independent check on the manufacturer’s claims. This can be provided to retailers such as Amazon.com which in August 2013 began requiring third-party verification of lasers sold on its website. UL can also assist with preparation of a manufacturer’s FDA report.
Click to read more...
These regulations, 21 CFR 1040.10 and 1040.11, require laser product manufacturers only to self-certify to the Food and Drug Administration that their products meet safety standards. Once the FDA’s Center for Devices and Radiological Health reviews and acknowledges the certification, the laser product can be marketed in the United States.
UL is providing a third-party, independent check on the manufacturer’s claims. This can be provided to retailers such as Amazon.com which in August 2013 began requiring third-party verification of lasers sold on its website. UL can also assist with preparation of a manufacturer’s FDA report.
Click to read more...
US: FDA "disapproves" of Wicked Lasers; stops imports
08 Dec 2010 -- Categories: Bans & Restrictions | Import seizures | Ways to reduce incidents | SLA news
Tech website Gizmodo reports that the FDA’s Center for Devices and Radiological Health (CDRH) sent a letter Nov. 3 2010 to Wicked Lasers, disapproving of “the quality control and testing program for all [Wicked] laser products.” This includes the well-known Spyder III Arctic, a nominal 1-watt, 445 nm blue handheld laser.
FDA cites eight items of noncompliance:
The restrictions will be lifted, FDA told Wicked, once “CDRH determines that you have established an adequate quality testing program, and you have submitted the required reports and report supplements.”
From Gizmodo. The full text of FDA’s warning letter to Wicked is after the link (click “Read more...”)Click to read more...
FDA cites eight items of noncompliance:
- Three of these items relate to a January 2006 letter which FDA says Wicked did not respond to.
- Four items relate to Wicked claiming in 2006 and 2008 that its lasers were sold for surveying, leveling and alignment (SLA) purposes; FDA says Wicked is not complying with restrictions on SLA lasers. (FDA has greater authority to regulate SLA lasers than it does to regulate general-purpose lasers).
- The final item objects to Wicked stating on its website that its products are “FDA Certified” when in fact the manufacturer certifies compliance to FDA, who reviews and files the certification documents.
The restrictions will be lifted, FDA told Wicked, once “CDRH determines that you have established an adequate quality testing program, and you have submitted the required reports and report supplements.”
From Gizmodo. The full text of FDA’s warning letter to Wicked is after the link (click “Read more...”)Click to read more...
US: New FDA publication on laser pointer hazards
27 Jun 2009 -- Categories: Easy availability | Import seizures | Ways to reduce incidents | SLA news
The U.S. Food and Drug Administration presented their views on the hazards of powerful laser pointers, in a webpage and in a downloadable PDF brochure. According to Cdr. Dan Hewett of the FDA’s Center for Devices and Radiological Health, “When used responsibly, lasers are safe. However, a powerful laser, used irresponsibly, is unsafe, particularly when misused as a toy or directed at people, vehicles or aircraft.”
FDA is especially concerned about laser pointers above 5 mW, and about aircraft incidents. “In 2008, pilots reported a total of 950 cases of laser light striking an aircraft or illuminating a cockpit....The distraction from flash blindness could cause a serious accident.”
Click to read more...
FDA is especially concerned about laser pointers above 5 mW, and about aircraft incidents. “In 2008, pilots reported a total of 950 cases of laser light striking an aircraft or illuminating a cockpit....The distraction from flash blindness could cause a serious accident.”
Click to read more...
