Home
A comprehensive resource for safe and responsible laser use
US: GAO report has three recommendations for FAA
The GAO began their study of the issue in November 2020. Their goal was to determine:
• "the extent to which [the U.S. Federal Aviation Administration] FAA and other federal agencies take enforcement action against those who point lasers at aircraft and challenges with investigations and reporting;"
• "public outreach efforts FAA and other federal agencies have taken to deter laser incidents, and what actions, if any, would strengthen these efforts; and"
• "options that stakeholders have identified to mitigate the effects of laser incidents, and the potential benefits and challenges to implementation."
FINDINGS
The following is from the "What GAO Found" summary of their findings:
"Aiming a laser at an aircraft can distract or disorient pilots and is a federal crime. The Federal Aviation Administration (FAA) investigates laser incidents, pursues civil penalties, and assists the Federal Bureau of Investigation (FBI) and U.S. Attorneys with investigations. Given the nature of laser incidents, FAA and federal law enforcement face difficulties identifying those involved. However, they have taken some enforcement actions, resulting in penalties ranging from $50 to $27,388 and sentences of up to 51 months, according to GAO analysis."
"To support incident investigations, FAA asks that pilots complete an incident questionnaire upon landing. However, FAA received responses for about 12 percent of the 8,221 laser incidents that occurred over a recent one-year period from 2020 to 2021. Reasons identified by FAA and others for the low response rate include the length of the questionnaire and its voluntary nature. Further, FAA does not consistently share collected information with law enforcement."
"In 2016, Congress required FAA to report quarterly on laser incidents, including data on civil and criminal actions. However, GAO found FAA’s reports to be incomplete. For example, GAO’s analysis shows 44 prosecutions from July 2016 through September 2020, when FAA reported only four. FAA officials said they do not routinely request data on the status of actions from other agencies and face challenges, such as access to this data. By not routinely seeking updates from agencies, FAA does not provide Congress with a complete picture of laser incident investigations and enforcement actions as required."
"FAA, FBI, and the Food and Drug Administration, which has regulatory authority over lasers, each conduct outreach to educate the public about laser incidents. These agencies were involved in an interagency group to address laser safety concerns until 2015 when the group dissolved. Since then, laser incidents have increased and identifying subjects remains difficult. FAA is well positioned to lead an interagency effort to explore re-establishing this group, given FAA’s responsibility for the safety of the national airspace."
RECOMMENDATIONS
The 46-page report recommended three actions to be taken by FAA:
1: "The FAA Administrator should determine what information from pilots and crewmembers would be most useful for investigating laser incidents, and how best to collect the information and to share it with law enforcement."
2: "The FAA Administrator should improve its quarterly reports to Congress on laser incidents by routinely seeking information from other agencies on related federal investigation and enforcement actions and disclosing, in those reports, any limitations with the data."
3: "The FAA Administrator should work with FBI and FDA to explore re-establishing an interagency working group on outreach to educate the public on the hazards of lasers and the illegality of aiming lasers at aircraft."
From the GAO webpage about the report. It includes a link to the full 46-page report.
COMMENTARY FROM LASERPOINTERSAFETY.COM
While the three recommendations are useful, we are disappointed that the GAO report did not make a primary recommendation that FAA should require pilot training on how to handle laser illuminations. In our view, this is the single most important safety step that FAA could take.
The report does discuss this, beginning on page 30 with this paragraph:
"Most stakeholders told us that training pilots on how to respond to laser incidents is an important mitigation strategy. For example, representatives from an organization representing law enforcement pilots told us that training pilots in responding to laser incidents is important because there will always be laser incidents. These representatives said that even with effective public outreach and enforcement activities, there would be people intentionally trying to harm aircraft and that in these situations, it is important for pilots to know how to react. Additionally, representatives from a group representing pilots told us they recommend airlines develop and incorporate a laser strike training module at a minimum of every 2 years." (emphasis added)
However, the GAO report simply lists some pilot training efforts. It does not say that FAA should mandate this.
LaserPointerSafety.com has also recommended that pilots be exposed to safe, simulated laser light during simulator training. This gives a flavor of what a bright light disruption can be and helps "inoculate" pilots so they know what to do, just as they train for other aviation emergencies.
US: 2 Watt "laser bongs" for sale
The laser beam is so intense it is a diffuse reflection hazard. The bong comes with 2 pairs of protective eyewear.
![]()
The founder of the company said in a November 2018 email to Mashable that the laser is not dangerous but can sting if you get your hand in it "kind of like a magnifying glass."
In addition to the 445 nm blue laser and protective glasses, the app-controlled bong also has a rotating bowl and color-changing LEDs.
The product has been in the works for some time. According to Gizmodo, a January 11 2018 Instagram video from Silicon Cali's founder demonstrated a prototype laser bong available for pre-order. On January 23 2018, he wrote on Instagram about shipping time: "It’s just dealing with the FDA regulation and all the other requirements for manufacturing and selling a high powered class4 laser product in the USA that take the time."
It is not known when the late fall 2018 version officially went on sale.
At the company's website, as of November 5 2018 there is no mention of FDA certification nor any picture of FDA-required warning labels, though there is a description of "turn key ignition." In the photo above, a small key can be seen inserted into the bottom of the bong. Federal law requires a key or similar lock-out device to prevent laser devices from being turned on by unauthorized users.
Laser bongs are a relatively old idea among persons interested in high tech and recreational drugs. A web search turns up a July 30 2009 post to Grasscity Forums, linking to a YouTube video of a prototype LaserBong (different product) made by the chief engineer of Wicked Lasers. The video is now unavailable at YouTube. Other YouTube videos still online show, for example, a June 4 2013 video of a person using a handheld blue laser to ignite bong material.
From Silicon Cali with additional reporting by Mashable and Gizmodo.
US: FDA recommends against using Laserworld and Ray Technologies laser projectors
Laserworld set up a special website, www.cdrh.info, with a statement and information from their viewpoint.
The International Laser Display Association published guidance for ILDA Members and others who are doing shows in the U.S. with Laserworld and RTI projectors.
US: FDA recalls certain X-Laser light show projectors
FDA listed the following actions:
X-Laser LLC will bring into compliance:
1. All purchasers and associated dealers of affected LLS projector models will be notified by mail and email of their failure to comply with the performance standard. The notification will follow the format and include the information required by 21 CFR 1003.21. Those that do not respond within 14 days will be notified a second time. Those not responding to the second attempt will be notified again every 6 months for the next 2 years. Non-responsive dealers will be ineligible for future orders.
2. All affected LLS projectors will be repaired by removing the auto and music modes from the dipswitch accessible settings, after which, these modes will only be accessible through the DMX connection. These actions, including transportation of the LLS projector, will be made free of charge.
3. All LLS projector models that X-Laser receives, regardless of purpose, will be checked for dipswitch accessible auto or music modes and repaired if needed.
4.Corrective actions will be completed within 120 days of receipt of this letter.
For further questions please call (866) 702-7768.
For additional details from X-Laser, click the “read more” link. Click to read more...
US: Nationwide recall of Santajoy Christmas laser lights sold at Walmart
On Dec. 22, 2017, Santajoy initiated a nationwide recall of Galaxy Holiday Laser Lights and Northern Lights Holiday Laser Lights. These laser projection products may incorporate a laser having a higher output than intended and fail to comply with FDA performance standard requirements (21 CFR 1040.10 and 1040.11). These higher-power lasers have the potential for eye injury.
Consumers who purchased any of the 5,254 units of Galaxy Holiday Laser Lights and Northern Lights Holiday Laser Lights sold by Walmart between August 1, 2017 and October 25, 2017 should stop using them and return them to any Walmart store for a full refund.
The Galaxy Holiday Laser Lights and Northern Lights Holiday Laser Lights were manufactured from May through September 2017 and distributed from August through October 2017. The affected products sold by Walmart can be identified by the packaging photos and UPC numbers shown below.
Santajoy voluntarily recalled these products after becoming aware that the product presented a potential safety hazard and has notified the FDA of this action. There have been no reports of injury related to the use of these products. Santajoy is notifying the public through this press release, and Walmart is accepting the return of these products for a full refund.
Walmart Stores Inc. distributed these products nationwide. Consumers with questions may contact Walmart via telephone at 1-800-Walmart from 7 a.m. to 9 p.m. CT Monday through Friday or online at www.corporate.walmart.com/recalls for more information.


Note: As of January 1 2018, neither laser was listed on the Walmart Product Recalls webpage. The product recall also did not appear to be at FDA’s recalls webpage or enforcement report webpage, as of January 1. The only online source on that date was the December 22 2017 Business Wire press release, or a few publications and news sources such as KCTV that reprinted the Business Wire press release.
US: FDA issues warning about laser toys
FDA gave these examples of laser toys:
- Lasers mounted on toy guns that can be used for “aiming”;
- Spinning tops that project laser beams while they spin;
- Hand-held lasers used during play as “light sabers”; and
- Lasers intended for entertainment that create optical effects in an open room.
According to the Consumer Update, “Toys with lasers are of particular interest to the FDA because children can be injured by these products. Because they are marketed as toys, parents and kids alike may believe they’re safe to use.”
The FDA had tips for safe use, including:
- Do not aim at persons or animals
- Do not aim at any vehicle, aircraft or shiny surface; or persons playing sports
- Children’s toy lasers should be Class I.
- Children should not be allowed to own or use laser pointers. Pointers are not toys.
- Do not buy or use any laser that emits more than 5 milliwatts.
- See a health care professional in case of a known or suspected laser eye injury.
The FDA’s health warning was referenced in numerous news and publication sources over the 2017 holiday season.
From the FDA Consumer Update, “Laser Toys: How to Keep Kids Safe”. FDA also linked to a 2015 FDA YouTube video on laser pointer safety.
For background, LaserPointerSafety.com has a series of webpages about laser toys which begin with a summary here.
US: FAA requests comments by Oct 31 2017 regarding form used to report outdoor laser operations
The form is filled out by commercial and professional users who want to operate lasers outdoors, including laser light shows, observatories, LIDAR operators, and satellite communications.
According to FAA, there have been about 400 laser operators who filled out the form. The agency also says it takes 4 hours to gather information needed for the form and to fill it out.
FAA needs to periodically review whether the form is useful and whether the 4-hour estimate is accurate. The deadline for comments is October 30 2017.
The August 31 2017 Federal Register notice states:
“In accordance with the Paperwork Reduction Act of 1995, FAA invites public comments about our intention to request the Office of Management and Budget (OMB) approval to reinstate a previously approved information collection. In order for the FAA to ensure safety it proposes to collect information from potential outdoor laser operators. The FAA will review the proposed laser activity against air traffic operations and verify that the laser operation will not interfere with air traffic operations.”
The notice details what information specifically is requested:
“You are asked to comment on any aspect of this information collection, including (a) Whether the proposed collection of information is necessary for FAA's performance; (b) the accuracy of the estimated burden; (c) ways for FAA to enhance the quality, utility and clarity of the information collection; and (d) ways that the burden could be minimized without reducing the quality of the collected information. The agency will summarize and/or include your comments in the request for OMB's clearance of this information collection.”
Form AC 7140-1 does not impact laser pointer users per se, but it can affect professional outdoor laser users.
- The most impacted are outdoor laser light show operators. They are required by the Food and Drug Administration to submit their shows to FAA, and to receive a “letter of non-objection” from FAA, before FDA will grant permission (a “variance”) for a show.
- All other outdoor users are requested to submit Form AC 7140-1, but are not legally required to do so. This is because FAA has no regulatory authority to restrict outdoor laser usage. There may be organizations such as NASA or observatories that have internal requirements to submit AC 7140-1 and receive a letter of non-objection. This is usually done in the spirit of cooperation and/or to help avoid liability issues in case of problems.
Additional information, including instructions on how to submit comments, is at the Federal Register notice webpage. LaserPointerSafety.com has a webpage with suggested corrections to Advisory Circular 70-1, and advice on filling it out.
US: UPDATED - FDA wants to allow only red laser pointers, calling all other colors "defective"

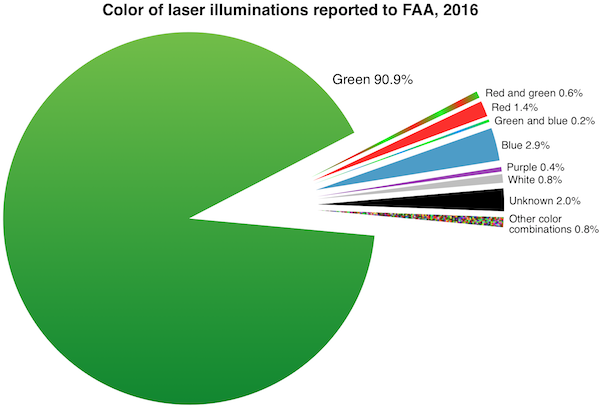
FDA’s primary concern is green lasers’ interference with the vision of vehicle operators including pilots. Green lasers are involved in over 90% of incidents where pilots reported to the Federal Aviation Administration (FAA) that they saw or were illuminated by laser light during a flight. (The charts below were added in January 2017 after the 2016 FAA final numbers came out.)
FDA is also worried about blue lasers which can have greater visibility to night-adapted eyes than red lasers of equivalent power.
Thus FDA is applying the “defective” label — giving them added authority over potentially injurious products — because of what they consider to be a well-known, established public safety hazard to operators of vehicles, aircraft and watercraft.
What FDA is trying to accomplish
FDA has two main goals:
1) “Turn back the clock” to the 1990s and early 2000s when almost all laser pointers were red. According to the agency, red light has the least interference with pilot vision, compared to equivalent-power green beams which can appear up to 28 times brighter. During this period there were dozens or low hundreds of reported laser/aviation incidents per year, compared with 7,703 incidents in 2015 and 7,442 incidents in 2016.
2) Make it much easier for customs and law enforcement to identify illegal laser pointers simply by their color. Red and orange-red laser pointers would be permitted; all others would be prohibited for general sales.
In addition, FDA sought to address requests from legislators including Senator Chuck Schumer (D-NY). After high-profile incidents, lawmakers have written to FDA, asking for a ban on green pointers due to their vision-blocking abilities being a risk to pilots and passengers.
Who would be affected
FDA’s proposed color-based prohibition would only affect the manufacture, importation and sales of laser pointer products introduced into commerce. Although pointers fall under the FDA’s “surveying, leveling and alignment” (SLA) control, only pointers as defined by FDA would be restricted to red. Standard SLA equipment would not be affected — they could use any color beam.
Individuals such as hobbyists who manufacture their own laser products for their personal use would be free from FDA laser product regulations. This is because such individuals would not be considered manufacturers by FDA.
Since federal law cannot control individual use or misuse, states and localities could impose their own regulations. (A few states and localities already have their own restrictions on use and/or possession; these are not currently based on the color of the laser.) One benefit of FDA’s proposal is that any new state and local laws could “piggyback” on FDA’s color-based restrictions. That would make it easier for local law enforcement to use color to easily identify whether a person possessed a prohibited or permitted laser.
FDA’s proposal and rationale was stated in draft amendments presented October 25 to an FDA advisory panel known as “TEPRSSC”.
Click to read more...
US: Coast Guard seeks FDA waiver; wants to use laser illuminators on helicopters
Currently, the U.S. Department of Defense is permitted to self-certify their laser equipment and usage. The DoD’s Army, Air Force and Navy agencies do not need FDA approval of their helicopter-based laser illuminators. However, the Coast Guard is part of the Department of Homeland Security, which does not have a self-certification waiver. The Coast Guard must currently apply for FDA approval.
On April 14 2016, Rep. Duncan Hunter sent a letter to FDA, asking that the Coast Guard be permitted to self-certify their laser systems. Hunter called FDA’s policy “onerous and burdensome”.
One issue may be that the helicopter-based video system already has low-light and infrared capabilities. Although the laser illumination can further enhance the image, it may not be considered a necessity for operations.
From Seapower magazine
US: Sen. Schumer gets new FDA leader to consider banning high-power green laser pointers
As of February 4, Califf’s nomination still awaited Senate approval.
Sen. Schumer, a Democrat from New York State, issued a statement saying “We’re only one month into 2016 and already there has been a green laser strike targeting aircrafts in the New York metropolitan area. We need to do something, and that is why I am pushing the FDA Commissioner nominee to act ahead of his confirmation. Green laser pointers have been a repeated danger to pilots across the country and I will continue to urge the FDA to use its authority and finally ban green, long-range, high-powered laser pointers once and for all.”
According to Newsday, there would be an exemption for professional uses. No additional details on the exemption criteria were available.
From Newsday (Note: accessing the article may require payment or answering survey questions)
US: UPDATED - FDA reminds consumers of laser pointer hazards
This is an update to a similar warning issued December 16 2010. It contains more specific guidance about how to tell whether a handheld pointer may be over the U.S. limit of 5 mW for a laser sold as a “pointer” or for pointing purposes.
Also, the 2015 version includes details about how to report potential laser injuries to FDA’s MedWatch medical device reporting system, and what information to include in the MedWatch report.
The full text of the FDA Safety Communication is below; click the “Read More…” link.
From U.S. FDA: December 22 2015 safety communication, and December 16 2010 safety notification
UPDATED February 19 2015 — The FDA also produced a video, entitled “Laser Pointer Safety”. The 3 1/2 minute presentation shows some of the hazards of laser pointers. It also gives recommendations on how to select and safely use laser pointers. From the FDA’s YouTube channel.Click to read more...
US: Sen. Schumer asks FDA to regulate sale of green lasers to public
The following is a press release issued by the Senator, followed by (after the “Read More:” link) a copy of the Senator’s letter to the FDA Commissioner:
Standing in the terminal at Buffalo Niagara International Airport in Cheektowaga, NY, U.S. Senator Charles E. Schumer today called on the Food and Drug Administration (FDA) to ban the sale of high-powered, long-range green laser pointers to the public. Schumer’s push comes on the heels of multiple incidents in which green lasers were pointed at aircraft and temporarily blinded and disoriented pilots mid-flight. This includes the recent incident two weeks ago when the pilot of a FedEx plane flying over Jamestown reported a green beam of light coming from a laser on the ground lighting up the aircraft. Schumer said that while it is lucky no one was harmed in the Jamestown incident or any other green laser attack, the federal government should act before a horrific event occurs, not after.
“Simply put: these green, long-range, high-powered laser pointers are a danger to our pilots and the hundreds of passengers whose lives depend on their eyesight and training. While we are very lucky the recent incident in Jamestown did not yield devastating results, we cannot sit idly by and wait for a horrific incident to occur before we act,” said Schumer. “That is why I am calling on the FDA to use its authority to regulate these dangerous devices. They're quickly becoming the weapon of choice for wrong-doers who want to harass our pilots and put passengers’ lives in jeopardy, and they should be banned before people are seriously hurt.”
Schumer said there has been a recent onslaught of green laser pointer attacks on aircraft that threaten the safety of pilots, passengers, and civilians on the ground. According to a USA Today report, the Federal Aviation Administration (FAA) recorded more than 5,300 laser strikes from January of this year through October 16, up from the more than 2,800 laser strikes reported in 2010. Schumer said numbers like these suggest a widespread misuse of the product and mean it should only be available to qualified professionals. According to the same USA Today report, between the night of November 11 and the morning of November 12, federal authorities reported 20 laser strikes on aircraft across the country, including the case in Jamestown. Schumer said the fact that the plane was flying more than 23,000 feet in the air shows how powerful these lasers are, and how dangerous they can be when they get into the wrong hands. As a result, Schumer is urging the FDA to ban the sale of high-powered, long-range green laser pointers to the public.
Laser pointers at one time were primarily used for presentation purposes in boardrooms and classrooms, they are now wildly available at trinket shops, flea markets, retailers and on the Internet, and are much more powerful. According to the FDA, laser pointers can be momentarily hazardous when staring directly at the beam. For pilots, these green lasers can cause flash blindness, a temporary or permanent loss of vision when the light-sensitive parts of the eye are exposed to an intensity of light they are not physically meant to handle. In addition, research suggests green lasers are more dangerous to the eye than red lasers because the light spectrum is more easily absorbed by the retina and more susceptible to damage. In fact, green lasers are considered to be more than double the strength of other colored lasers and can travel for miles, according to many media reports and health and aviation experts. Schumer there are certain types of lasers for which manufacturers must obtain FDA permission before they can be sold in the U.S., and green lasers should be included in that category so they are only sold to professionals, rather than would-be pranksters.
Because the FDA has the authority to regulate these lasers and their manufacturers, Schumer is strongly urging the federal agency to make high-powered, green laser pointers unavailable for public sale; they should be restricted to those with a specific professional purpose. Schumer said that while perpetrators convicted of pointing a laser at a plane can be sentenced to 20 years in prison and a $250,000 fine, they are often hard to track down following an incident. Because the products are merely required to have a warning label, Schumer said more must be done to limit public availability in order to protect public health and safety.
Schumer was joined by Adam Perry, Aviation Committee Chairman at the NFTA, Kimberly A. Minkel, NFTA Executive Director, and George Gast, NFTA Police Chief.
“I applaud Senator Schumer for his efforts to ensure the safety of our aviation industry,” said Kimberley A. Minkel, Executive Director of the Niagara Frontier Transportation Authority. “I hope the FDA responds to the senator’s request in a manner that will make it much more difficult for laser pointers to be available. Lasing is a serious crime that poses an imminent threat to aviation safety and could result in a pilot losing control of their aircraft, thus potentially causing mass casualties.”
Previously, the FDA has noted concern about the increased availability of some laser products. According to a March 2013 study by the National Institute of Standards and Technology (NIST), green lasers generate green light from infrared light, from which the eye cannot protect itself. In that NIST report, the agency noted that ideally, the device should be designed and manufactured to confine the infrared light within the laser housing. However, according to the NIST results, more than 75 percent of the devices tested emitted infrared light in excess of the Code of Federal Regulations (CFR) limit.
Schumer said in New York incidents of green lasers pointed at aircraft have been numerous and significant. In addition to the most recent one in Jamestown, there were 39 laser incidents between January 1, 2015 and May 15, 2015 in New York City alone. In 2014, there were 17 green laser incidents out of a total 19 laser incidents at JFK airport; 37 green laser incidents out of a total 41 laser incidents at LaGuardia Airport; 20 green laser incidents out of a total 28 at Newark.
There are four major hazard classes (I to IV) of lasers, including three subclasses (IIa, IIIa, IIIb). The higher the class, the more powerful the laser. Consumer laser products include classes I, II and IIIa and lasers for professional use may be in classes IIIb and IV. Laser pointers are included in Class IIIa. The FDA requires warning labels on most laser products, including the power output and the hazard class of the product. Some lasers are strictly for use by medical, industrial or entertainment professionals and can only be used by a person with a license and training.
A copy of Senator Schumer’s letter to the FDA appears below: [click on the “Read More…” link]
Click to read more...
US: NY senator wants FDA to ban green laser pointers
Schumer made the announcement at a Sunday press conference in his Manhattan office, along with four commercial airline pilots who had been illuminated by laser light. One pilot, Gabe Rubin, said he knew of a pilot who “suffered severe eye damage from a green laser pointer [and] will never fly again.”
Schumer said “Green lasers are the weapons of choice being used for evil purposes. We know terrorists are always looking for areas of weak points.”
He is focused on green pointers because they are apparently preferred by pranksters because the green light travels farther, and “because the light spectrum of green is more easily absorbed by the retina and then causes more damage”, according to the senator.
In 2012, Schumer wrote a letter to the U.S. FDA saying that laser pointers’ power should be less than the current 5 mW limit, that FDA should restrict more powerful Class 3B (5-500 mW) and Class 4 (500+ mW) lasers, and that FDA should require warning labels about aiming at aircraft.
From Newsday and CBS New York. The text of Sen. Schumer’s press release is below (click the “Read more…” link).
Click to read more...
US: FDA issues guidance on lasers in toys; wants Class 1 only
In addition to toys with visible beams that are dimmer than laser pointers, the other type of children’s Class 1 laser products are those that have internal, inaccessible lasers. For example, the laser inside a CD or DVD player device is often Class 3B — well above 5 mW. But because the beam cannot be accessed under normal conditions, the entire device is Class 1.
What laser toy products are included
FDA’s guidance is for “children’s toy laser products”, defined by the agency as “a product primarily used as a toy that is manufactured, designed, intended or promoted for novelty or visual entertainment use by children under 14 years of age.” It does not include “laser products that are used in professional or academic settings that may be used by children (for example, laser printers, CD players, educational and science kits).”
To determine if a laser product is a toy, FDA takes into account factors such as the promotion and product graphics (for example, if children are shown playing with the product), the location of sales such as toy stores or websites, and whether features or the nature of the product may indicate it is intended for children.
The agency gives examples of children’s toy laser products:
- Lasers mounted on toy guns that can be used for “aiming”
- Spinning tops that project laser beams while they spin
- Hand-held lasers used during play as “light sabers”
- Dancing laser beams projected from a stationary column with bright colors or pictures on the box that might appeal to children
- Lasers intended for entertainment that create optical effects in an open room with bright colors or pictures on the box that might appeal to children.
US: Laser show company has variance revoked for unauthorized audience scanning
On July 24 2014, the Food and Drug Administration sent a letter to David Fleenor of Epic FX, Inc. of Phoenix, Arizona. It stated that videos posted on the epicfx.com website “documents audience scanning with Class IIIb and/or Class IV lasers. Although much of the audience scanning was done with fanned beams, your projector is not designed nor reported for safe audience scanning. Your variance prohibits audience scanning. Any laser beams projected into the audience directly or indirectly is considered audience scanning. This is in violation of Condition 5 of your variance.” [The page has since been removed, and returns a 404 error.]
Click to read more...
US: FDA issues guidance to public on high-powered laser "pointers"
According to the agency’s information, published July 5 2014, FDA requires that manufacturers of handheld, battery-powered lasers limit the power of the laser light to five milliwatts or less. The label must state the power and the hazard class.
The guidance tells the reader not to purchase handheld, battery-powered lasers above 5 milliwatts “unless the manufacturer has an approval from FDA (called a ‘variance’) to allow the purchase.” Otherwise, the sale is illegal, according to the agency.
They also warn against aiming lasers at eyes, at reflective surfaces into eyes, or at the operator of aircraft, watercraft, or vehicles.
From the FAA Basics webpage entitled “Does FDA regulate these new powerful laser ‘pointers’ and are they hazardous?”
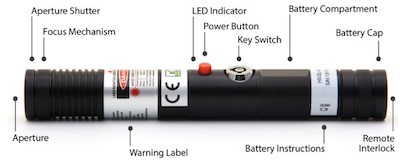
US: Review of 1-watt blue laser with US-required safety features
The SKY Technologies Blue Handheld includes a keyswitch, 3-5 second emission delay, remote interlock, and a shutter to cut off the beam, as required by FDA regulations enforced by the agency’s Center for Devices and Radiological Health (CDRH). Under current (May 2014) law, the laser appears to be legal for sale and use in the U.S., assuming the manufacturer also submitted a proper Laser Product Report and has filled all other FDA/CDRH import and paperwork obligations.*
US: FDA proposes defacto ban on selling pointers, handhelds above 5 milliwatts
Although the agency did not give a reason, such bans have been imposed in other countries in response to climbing numbers of laser illuminations of aircraft as well as reports of eye injuries caused by higher-powered consumer lasers.
The proposal would not make it illegal to own or responsibly use portable, battery-powered lasers of 5 mW or more. However, manufacturers could not make or sell these into general commerce in the U.S.
The agency will accept comments for 90 days (until August 2 2014) on the new proposal. FDA will then review the comments. Based on whether it believes any objections or suggestions are valid, the agency could put the guidance into effect (thus imposing their new interpretation), could submit a revised proposal, or could withdraw its proposal.
What lasers are covered by the proposed 5 mW limit?
FDA does not have direct authority over battery powered portable lasers. For example, the words “pointer” and “handheld” laser do not appear in U.S. laser regulations 21 CFR 1040.10 and 1040.11.
Therefore, to regulate these lasers, the May 5 draft proposes an extension of the FDA’s existing authority to regulate surveying, leveling and alignment (“SLA”) lasers. In the May 5 proposal, FDA asserts that the existing definition of SLA lasers also can applied to lasers with the following design characteristics:
- Compact size (i.e. small, lightweight)
- Battery power
- Ergonomic design to permit hand-held use
- An aperture in the laser product's protective housing to transmit laser emission into open space
- Portability to permit use in open spaces or in unrestricted environments
- Features that utilize the laser’s straight line emission for surveying, leveling, or alignment
According to the FDA, these types of lasers would be affected by the new 5 mW limit:
- Laser pointers
- Levels
- Tools incorporating laser guides
- Gun sights
- Target designators
- Night vision illuminators
- Visual disruptors
What lasers are NOT covered by the proposed 5 mW limit?
The FDA's proposed 5 mW limit would NOT apply to lasers with the following design characteristics:
- Predictable, stable power input and output
- High quality power supply and/or power conditioning components
- Adjustability of power and wavelength
- Design that facilitates remote actuation
- Non-portability
- Hard wire connection to power mains
From the FDA’s Surveying, Leveling, or Alignment Laser Products - Draft Guidance for Industry and Food and Drug Administration Staff webpage, published online May 2 2014. This webpage includes the procedure for submitting comments to FDA.The FDA’s PDF version of the draft guidance document is here.
Editorial comment from LaserPointerSafety.com: We have previously published our opinion disagreeing with the FDA’s interpretation of “SLA” lasers. The existing regulations are clear on what constitutes “surveying, leveling or alignment” (SLA) lasers. While we understand the FDA’s intent, in our view, they are going about it the wrong way. They are essentially “making it up” by adding characteristics (size, battery power) which are in no way derived from the clear, existing definition of SLA lasers. As support of this position, we have not found any surveying, leveling or alignment lasers which look the same as the majority of laser pointers and handhelds. This topic is discussed in much greater detail on our page describing FDA authority over laser pointers and handheld lasers.
US: Underwriters Labs offers third-party testing for laser pointer sellers
These regulations, 21 CFR 1040.10 and 1040.11, require laser product manufacturers only to self-certify to the Food and Drug Administration that their products meet safety standards. Once the FDA’s Center for Devices and Radiological Health reviews and acknowledges the certification, the laser product can be marketed in the United States.
UL is providing a third-party, independent check on the manufacturer’s claims. This can be provided to retailers such as Amazon.com which in August 2013 began requiring third-party verification of lasers sold on its website. UL can also assist with preparation of a manufacturer’s FDA report.
Click to read more...
US: FDA asks Customs' help on illegal imports of laser pointers
Using Form 2877, the importer must submit information on each shipment and must affirm that the products comply (or do not comply) with FDA laser regulatory standards. But if a small package omits Form 2877 and is mislabeled (not using the word “laser”), this is an attempt to evade FDA and Customs. FDA specifically notes that such single-package Section 321-type imports do not meet the FDA’s criteria for enforcement discretion for personal importation.
Lasers that FDA is interested in include laser pointers, laser gun sights, laser levels, laser light shows, laser pointer key chains, veterinary laser products, laser illuminators and similar products. If a shipment does not meet FDA requirements, it can then be detained by the FDA and would not be allowed into the country.
From STR Trade Report. Thanks to New Aje Lasers for bringing this to our attention.
US: FDA warns parents of dangers of laser toys; issues draft guidance
In an August 6 2013 press release and Consumer Health Information article, FDA warned parents that lasers operated unsafely can cause serious eye injuries and even blindness. FDA said injuries from lasers can go unnoticed for days or weeks since there is no pain. But vision can slowly deteriorate over time, eventually causing permanent eye damage.
FDA gave the following as examples of children’s toy laser products:
- Lasers mounted on toy guns that can be used for “aiming”;
- Spinning tops that project laser beams while they spin;
- Hand held lasers used during play as “light sabers”;
- Dancing laser beams projected from a stationary column; and
- Lasers intended for entertainment that create optical effects in an open room.
Interestingly, the FDA’s press release and article gave tips on safe usage, including not aiming at car drivers or sports players -- but did not say that it is unsafe and illegal to aim at aircraft.
On August 7 2013, FDA issued draft guidance for industry on minimizing the risk of lasers in children’s toys. Comments are invited within 90 days of the Federal Register publication of the guidance, or by November 4 2013. The draft guidance is reprinted below.
Click to read more...
US: FDA proposes amending Federal laser manufacturer regulations
The proposal was issued in the Federal Register on June 24 2013. The public may send comments to FDA until September 23 2013. FDA will then evaluate the comments, make any changes as a result, and at a future date will put the amendments into effect.
For consumer lasers, the most significant proposal is to create a new category of specific purpose lasers, “children’s toy laser products.” FDA says these could include lasers intended for creating entertaining optical effects, dancing laser beams projected from a stationary column, spinning tops which project laser beams, or lasers mounted on toy guns for “aiming.” FDA defines such toys as “a product that is manufactured, designed, intended or promoted for use by children under 14 years of age.”
The laser inside such a toy would be restricted to Class I (less than 1 mW for visible light). This is because FDA is concerned that if the toy were broken or disassembled, a higher power laser could harm a child.
Click to read more...
US: FDA now recommending aircraft/vehicle caution label
“CDRH recommends (but does not require) labeling on your product that cautions the purchaser with following or similar language: “CAUTION - LASER LIGHT IS BRIGHT AND BLINDING - DO NOT SHINE AT AIRCRAFT OR VEHICLES AT ANY DISTANCE”.
While FDA can require the familiar labels warning against laser eye and skin hazards, FDA does not have statutory authority to require labels for non-health hazards such as laser distraction or temporary flash blinding. Thus, the agency is only able to recommend -- but not require -- the new aircraft/vehicle caution label.
News of the action came in a December 7 2012 FDA email sent to parties including LaserPointerSafety.com. According to CDRH’s Daniel Hewett, the action applies to all “SLA products.” FDA/CDRH considers that laser pointers and handheld lasers are a subset of such Surveying, Leveling and Alignment laser products. SLA lasers are one of the three laser product uses which FDA can regulate under 21 CFR 1040.10 and 1040.11; the other two are medical and demonstration (education/light show) laser product uses.
From a Dec. 7 2012 FDA email. Thanks to Daniel Hewett for bringing this to our attention.
US: Sen. Schumer asks FDA to overhaul laser regulations
From the Associated Press via the Wall Street Journal. To read the text of Schumer’s letter to the FDA, and his press release, click the Read More link below.
Click to read more...
US: FDA updates "Red List" of banned laser importers and products
- The laser product does not have a permanently attached warning logotype label;
- The laser product output exceeds 5 milliwatts;
- The laser product fails to contain certification or identification information either on the product or in the instructions for use;
- The laser product fails to contain instructions for safe use;
- The product class or output information on the laser product's warning logotype label is different from that in the instructions for use; and/or
- A product report for the laser product has not been submitted.
Products which can be Detained Without Physical Examination (DWPE) include laser pointers, laser gunsights, laser pens, laser light show projectors, laser special effects, laser levels, toy guns with lasers, laser pointer key chains, and similar products.
It is unclear what effect the FDA’s import restrictions have on supplies. For example, the well-known company Wicked Lasers is listed multiple times as being banned from importing “All laser products and all products containing lasers.” However, a company representative on December 22 said that Wicked does ship to the U.S. and there should be “no issues getting a laser into the U.S.”
Violating companies are listed as follows.
- Canada: 2 companies shipping from a total of 3 addresses
- China: 51 companies shipping from a total of 57 addresses
- Hong Kong: 9 companies shipping from a total of 9 addresses
- Japan: 1 company shipping from a total of 2 addresses
- Taiwan: 25 companies shipping from a total of 28 addresses
- United Kingdom: 1 company shipping from 1 address
From the December 20 2011 update to the FDA Red List
US: 3 sheriff's officers charged with illegal laser sales
92 laser sights and 74 automatic machine guns were ordered between Sept. 2008 and January 2010 on Lake County letterhead and purchase orders. The officers paid for the products with personal funds. The amount earned from Internet resales was not stated, although the three officers were also indicted for understating their personal income by a total of $387,000.
The laser products came from Insight Technology Inc. and Laser Devices Inc. The 92 restricted laser sights were purchased for approximately $1000 to $1400 each and were sold on eBay for around $2800 to $4200 each. A special agent of the U.S. Food and Drug Administration (which regulates laser devices) made an undercover purchase as part of the evidence-gathering process in the case.Click to read more...
US: FDA warns of risk from high-powered lasers
The announcement, dated a week before Christmas said “high-powered laser pointers” are “illegal and potentially dangerous.... The FDA wants to make consumers aware that they should not buy these lasers for themselves or as gifts for others.”
The announcement noted that “many eye injuries from laser pointers go unreported.” Of reported injuries, FDA said in 2010 they were aware of three incidents involving children playing with laser pointers. One of these caused damage “from reflected beams after directing a 150 mW laser pointer into a mirror.”
FDA also described incidents of pilots experiencing temporary flash-blinding. In 2009, pilots reported 1500 incidents; in the first 10 months of 2010, there were 2321 incidents. FDA noted in boldface that “Using a laser to illuminate aircraft is a federal crime.”
FDA listed five recommendations:
- Do not let children own or use laser pointers.
- Do not buy any laser pointer over 5 mW
- Do not aim laser pointers at any “person, pet, vehicle or aircraft” either directly or through reflections.
- If you own a laser pointer over 5 mW, “dispose of it safely according to local environmental protection guidelines.”
- If you are injured, see your eye doctor
The complete safety notification can be found on this FDA webpage, and is also reprinted here (click on the “Read more...” link).Click to read more...
US: FDA "disapproves" of Wicked Lasers; stops imports
FDA cites eight items of noncompliance:
- Three of these items relate to a January 2006 letter which FDA says Wicked did not respond to.
- Four items relate to Wicked claiming in 2006 and 2008 that its lasers were sold for surveying, leveling and alignment (SLA) purposes; FDA says Wicked is not complying with restrictions on SLA lasers. (FDA has greater authority to regulate SLA lasers than it does to regulate general-purpose lasers).
- The final item objects to Wicked stating on its website that its products are “FDA Certified” when in fact the manufacturer certifies compliance to FDA, who reviews and files the certification documents.
The restrictions will be lifted, FDA told Wicked, once “CDRH determines that you have established an adequate quality testing program, and you have submitted the required reports and report supplements.”
From Gizmodo. The full text of FDA’s warning letter to Wicked is after the link (click “Read more...”)Click to read more...
Worldwide: Major laser seller adds aircraft warning
WARNING: DO NOT SHINE YOUR LASER AT AN AIRCRAFT
Shooting a laser at an aircraft is considered a felony in the U.S.
The new label is being introduced in September 2009 by Wicked Lasers. They have a significant presence on the Internet, marketing a wide range of lasers for pointing, burning/cutting, and general purpose uses.
US: New FDA publication on laser pointer hazards
FDA is especially concerned about laser pointers above 5 mW, and about aircraft incidents. “In 2008, pilots reported a total of 950 cases of laser light striking an aircraft or illuminating a cockpit....The distraction from flash blindness could cause a serious accident.”
Click to read more...
