Home
A comprehensive resource for safe and responsible laser use
US: Review of 1-watt blue laser with US-required safety features
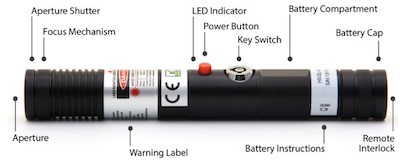
The SKY Technologies Blue Handheld includes a keyswitch, 3-5 second emission delay, remote interlock, and a shutter to cut off the beam, as required by FDA regulations enforced by the agency’s Center for Devices and Radiological Health (CDRH). Under current (May 2014) law, the laser appears to be legal for sale and use in the U.S., assuming the manufacturer also submitted a proper Laser Product Report and has filled all other FDA/CDRH import and paperwork obligations.*

The blog posted videos showing the SKY laser popping balloons and burning through a LEGO brick in 15 seconds.
Rees’ review concluded: “A laser with this kind of power is certainly not a toy and most people do not really have a worthwhile use for it either. But I equate it to a high-tech, modern day equivalent to the M80′s I used to ‘play’ with as a kid. Take that for what it is worth, I know these lasers can blind and are a felony to aim at aircraft, but when used responsibly can be fun and educational as well.”
From the Gadgeteer blog
*NOTE: On May 5 2014, FDA published its intent to define compact, battery-powered handheld portable lasers as “surveying, leveling or alignment” or “SLA” lasers. This would restrict such lasers to 5 milliwatt maximum output. The SKY laser, at 1000 milliwatts, would be effectively banned if the proposal becomes FDA Guidance. However, FDA’s current authority as of May 2014 indicates that FDA does not have the authority to regulate general-purpose handheld lasers, as long as 1) the laser is not sold as a “pointer” or for pointing applications, 2) the laser has all required safety features (as the SKY laser appears to have) and 3) the Laser Product Report, importation form, and other paperwork have been correctly submitted to FDA.